New from Myloc
Read our latest news, press releases and client case articles.

Morrow Batteries’ Gigafactory Leverages Digitized Logistics with Myloc Construction
Streamlining Project Supply Chains – Morrow Batteries, Metier and Myloc Construction. Morrow Batteries is creating an innovative industrial site in southern Norway built on several cornerstones; a locally secured renewable energy supply, access to a deep sea port to ensure international freight capabilities and a long-term development concept. Eyde Energy
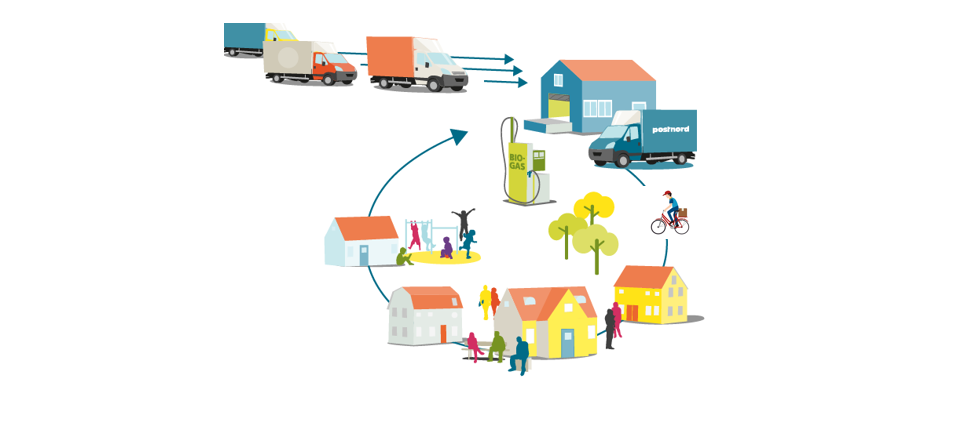
Thanks PostNord and Kungsbacka Municipality for our cooperation on Coordinated Goods Distribution!
Eight years ago, in 2016, Kungsbacka Municipality started a pioneering project around introducing Coordinated Goods Distribution to the municipality’s various operations. They had seen a need to streamline their product deliveries and goods receipts. At the same time, opportunities were found here to increase safety and control, reduce environmentally hazardous

MDR and Assistive Technology according to Myloc MedTech
Is your assistive technology organization in compliance with MDR? Do you and your manufacturers keep track of which of your products are classed as medical devices, including attachments and spare parts? Can your current system support identify and manage all the information required by the new law? The Medical

Myloc Construction tip: Knowledge seminar on digitized construction logistics with LTU
Seminar on construction logistics coming up this spring. Join the discussion when the industry meets at Luleå University of Technology to talk about the trend towards more digitalized construction logistics and how we can create safer and more efficient construction sites. Myloc Construction’s own Anders Eklund is one of the

Bravida Förbifart Akalla and Myloc Construction
Sweden’s longest motorway tunnel is taking shape Myloc Construction has become central partner for Bravida during their work at the new tunnel E4 Förbifart Stockholm. In this giant project led by the Swedish Transport Administration, Bravida is responsible for installing the electrical supply, lighting, plumbing, VA and fixed extinguishing systems.

The Stavanger hospital project – The biggest construction project in Norway
This article is part of a series where we at Myloc Construction visit landmark construction projects we are involved in. At the same time, we want to highlight interesting and innovative projects within the construction industry. One of Norway’s largest construction projects is currently underway in Stavanger. A university hospital

Have you heard about Hållbar Byggbransch?
The banking initiative Hållbar Byggransch rolls out on November 27. This means that the banks will change their conditions for granting credit when financing construction. The goal is to clean up the industry and prevent financial irregularities that have become common in Sweden in the last few years. Hållbar Byggbransch

The Assistive Device Center in Eastern Skåne has chosen Myloc MedTech for assistive device logistics
In Sweden, there hasn’t been much competition in the market for public assistive device logistics systems in the last 10 years. As a consequence, a significant portion of potential development has been lacking. The Assistive Device Center in Eastern Skåne is a collaboration between 11 municipalities in

Exploring Opportunities with the UN Tender Program at the Nordic UN Procurement Seminar
At Myloc, we have an exciting history with UNHCR, and we are eagerly looking forward to participating in the upcoming Nordic UN Procurement Seminar. This event offers a unique opportunity to learn more about the UN Tender Program and network with relevant stakeholders, and see how our system solutions can

Breeam – Promotion of environmental certification with Myloc Construction
What does Breeam mean? In the ever-growing construction industry, sustainability is no longer a wish but a necessity. A prominent tool for achieving this is BREEAM, which stands for “Building Research Establishment Environmental Assessment Method”. Originally developed in the UK in the 1990s by BRE (Building Research Establishment), BREEAM has
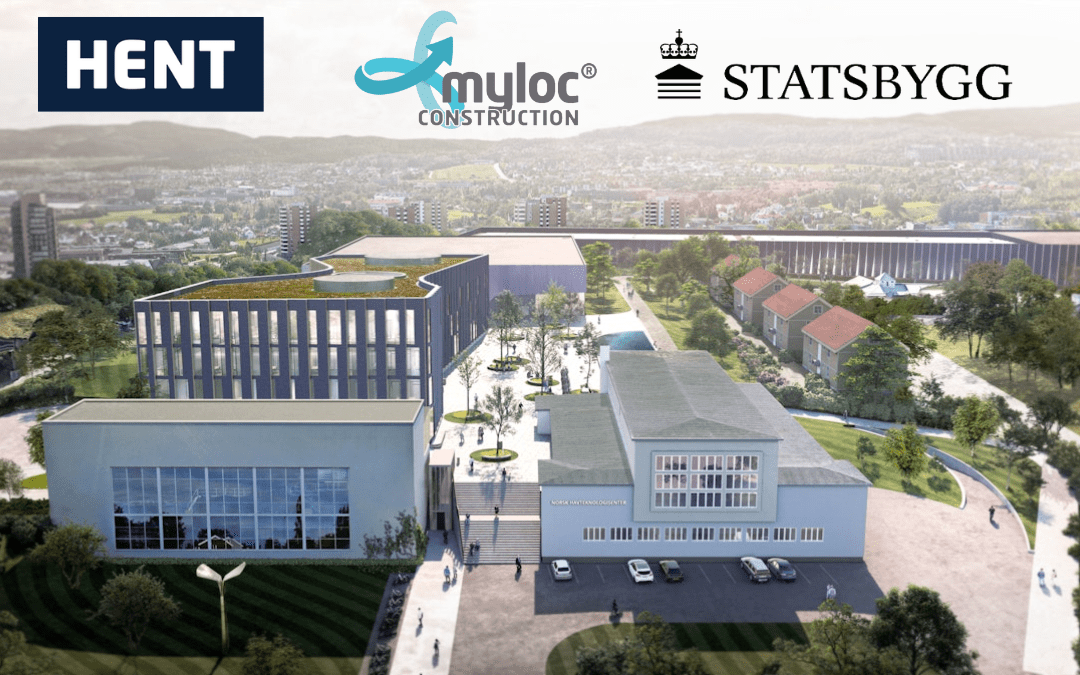
HENT and Statsbygg engage Myloc to solve construction logistics at The Norwegian Ocean Technology Center
Smart construction logistics paves the way for scientific innovation “The Norwegian Ocean Technology Center is a future research facility which with it’s size and technology will be almost unique in the world. Therefore, it is with pride, enthusiasm and humility that we take on this assignment. We are happy

Manage risks and secure flow in your giga project with Myloc Construction
Join us in our webinar series specialized to help you Turn Project Risks to Catalysts for Success with a Digitized management Platform in your mega project. Industrializing project logistics management Management and coordination of logistics and resources in industrial projects are increasingly fundamental for tight time and cost plans. Myloc,
